A Superficial Scrutiny of Exosomes, their Role in Carcinogenesis and their Therapeutic Potential
Amy Worrall TCD School of Medicine, Trinity college Dublin, Dublin 2.
ABSTRACT
Exosomes are nano-sized endosomal vesicles with a lipid bilayer membrane and a variable selection of cytosolic contents that are released by cells into extracellular fluids. They are found in most bodily fluids and are mediators of intercellular connections, signal transmission, and cell-to-cell communication. The exosome system, however, is also susceptible to dysregulation and carcinogenesis. An aberrant exosome system occurs in a tumour microenvironment, promoting survival, maximising local invasion and facilitating metastasis. The mechanisms by which exosomes facilitate carcinogenesis are multifactorial, with many elements facilitating the promotion of pro-tumour stroma, as well as transportation of tumour nucleic acids or factors enabling metastatic spread. Exosome detection has the potential to become a relevant biomarker in both diagnosis and prognostication through acquiring a liquid biopsy (i.e. blood sampling). Significant investigation into the use of the exosomes as an innovative drug delivery mechanism is also underway..
Article
AN INTRODUCTION TO EXOSOMES
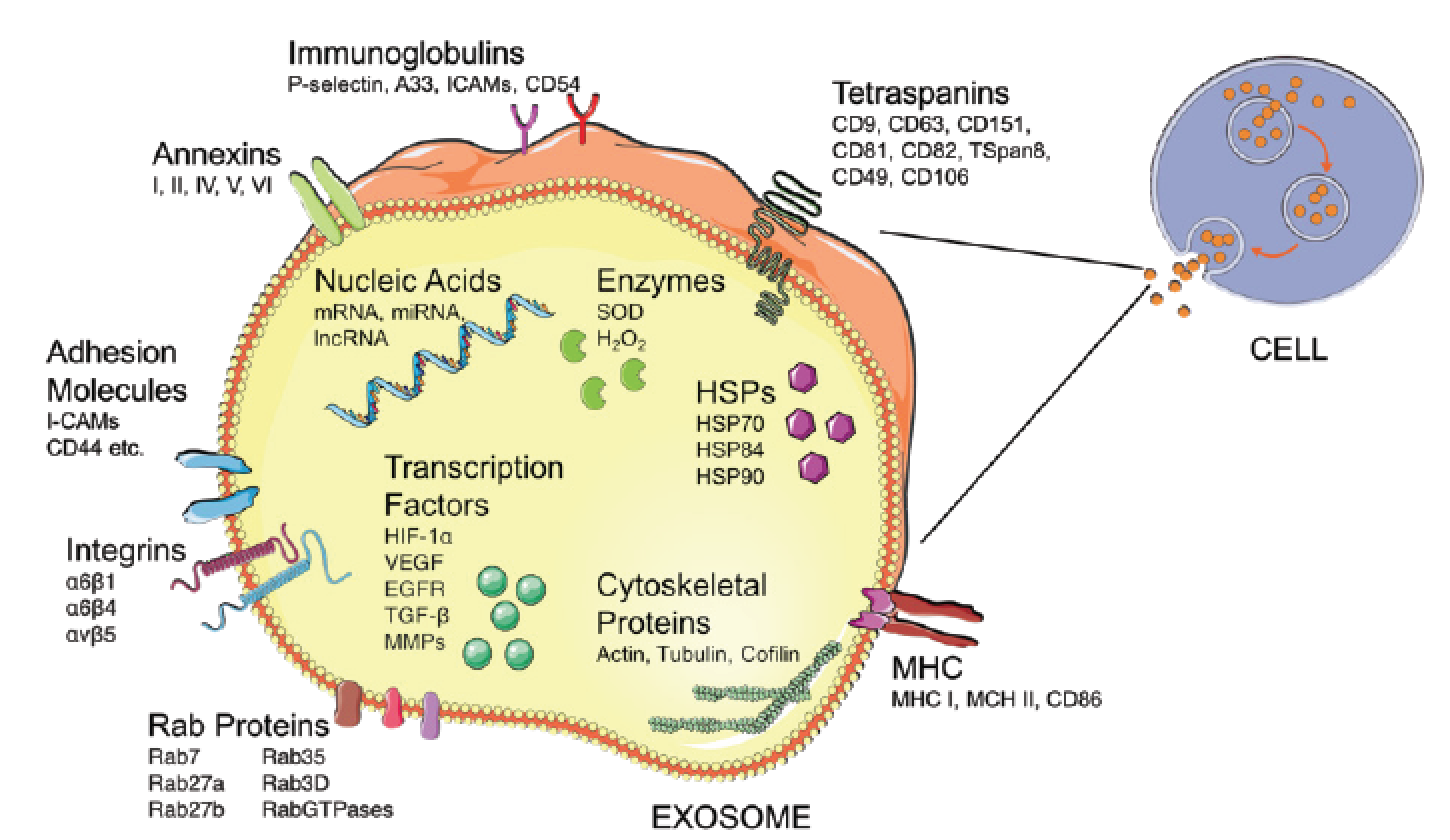
Exosomes are nano-sized vesicles (30-100nm) released from most mammalian cells including haematopoietic cells, endothelial cells, immune cells, platelets, neurons, muscle and tumour cells1,2 (Figure 1). They are comprised of a cholesterol and ceramide-rich lipid bilayer membrane, with a variable selection of cytosolic proteins, membrane-bound proteins and nucleic acids, such as such as Messenger RNA (mRNA), Micro RNA (miRNA), Non-Coding RNA (ncRNA) and Long Non-Coding RNA (lncRNA)3. The protein contents and identifying markers of exosomes differ significantly based on the origin cell they are released from and largely their contents are cytosolic in nature4. Normally, exosomes do not contain any intranuclear, mitochondrial, endoplasmic-reticular or golgi-apparatus proteins of the origin cell5. These exosomal cytosolic proteins range from messenger proteins, metabolites, transcription factors, to inflammatory cytokines6. Notably, the exosomal lipid bilayer membrane is a distinct membrane of endosomal origin, improving detection potential5.
Exosomes are released from cells by direct and indirect pathways from larger multi-vesicular bodies of endosomal origin, or through direct fusion with the plasma membrane1. The release can be passive, as part of normal cellular function, or selective, in response to cellular changes7. The Endosomal Sorting Complexes Required for Transport (ESCRT), tetraspanin, Rab proteins, and ubiquitylation machinery are thought to regulate the sorting, transport, and release of exosomes1.
FIGURE 1: The contents and surface constituents of an exosome. This figure is not an exhaustive representation of exosomal contents, and contents vary depending on original cell type that released each exosome. Figure adapted from various sources8,9 and produced by the author.
Exosomes are taken up by local or distant cells and, depending on the exosomal contents, modulate the recipient cell. Exosomes maintain their functionality and activity through the expression of both Major Histocompatibility Complex class I and class II (MHCI and MHCII) molecules on their vesicular surface10. They also express phosphatidylserine on the outer surface, facilitating their incorporation of scavenger proteins and integrins and their fusion with host cells11. Heparan sulphate proteoglycans, a normal endogenous macromolecular uptake system, on target cell membrane surfaces facilitate the internalisation of exosomes in target cells12.
EXOSOMAL FACILITATION OF CARCINOGENESIS, TUMOUR PROGRESSION, AND METASTASIS
Metastatic disease has been postulated as the direct, vascular, and lymphatic dissemination of cancer cells to distal tissues through the movement and transport of whole cells. Exosomes a are novel hypothesis for horizontal metastasis by the transfer of metastatic and carcinogenic traits, known as the ‘genometastasis hypothesis’. The process is believed to occur through the delivery of nucleic acids, carcinogenic proteins and modulators into target cells inducing metastatic change13. Exomic function, as well as protein and messenger content within the exosomes most likely vary in normal physiology and in pathology.
Exosomal Nucleic Acids and Genetic Information Transmission
Nucleic acids, including miRNA, lncRNA, and even mitochondrial DNA, all have the capability of inducing a pro-metastatic response fostering a tumour microenvironment7. Nucleic acids can be protein-bound in extracellular fluid or encapsulated in exosomes. miRNA derived from tumour exosomes are stable and fully functional in recipient cells and induce downstream transcription, as well as protein translation in the host cell7. Specific and unique tumour nucleic acids have been identified, such as the mutant mRNA for EGFR variant III (EGFRvIII) in glioblastoma exosomes14.
The tumour-specificity of these nucleic acids has reinforced the theory that exosomes are transferable transcriptomes for metastatic spread15. miRNA-200, containing exosomes derived from breast cancer cells, may confer metastatic carcinogenesis in distant non-tumour cells, and induce metastatic capability in non-metastatic tumour cells7. miR-21 and miR-29a from lung carcinoma exosomes binds Toll-like Receptors, inducing a pro-metastatic response in recipient cells with the release of Interleukin-6 (IL-6) and Tumour Necrosis Factor (TNF-α)16.
lncRNAs, such as linc-VLDLR (extracellular vesicle lncRNA) or linc-ROR (regulator of reprogramming), shuttled in hepatic carcinoma tumour exosomes, have been shown to promote chemoresistance in target malignant cells3. Similarly, lncRNA for HOX Transcript AntiSense RNA (HOTAIR) targets the p21 signalling pathway in lung cancers and transfers resistance to the chemotherapeutic drug cisplatin17. To date, dysregulation of RNAs has been implicated in carcinogenesis in almost every cancer-exosome system and as such is a target for anti-tumourigenic therapy18.
Invasion, Adhesion and Homing by Exosomes
Heparan sulphate proteoglycan cell surface macromolecules on target cells enable internalisation of tumour exosomes into recipient cells, assisting tumour progression. Cleaving of heparan sulphate proteoglycans reduces exosome intake, and tumour metastases12. Epidermal Growth Factor (EGF), and some mutant variants and fusion variants, have been documented in lung cancer exosomes17, and the presence of high levels of Epidermal Growth Factor Receptor (EGFR) in breast cancer target tissues, possibly reinforcing metastatic spread19. A splice variant of Cluster of Differentiation-44 (CD44), CD44 variant 6 (CD44v6), an abundantly expressed adhesion molecule, has been implicated in metastatic spread in both colorectal and pancreatic cancer, through binding with a constitutively expressed exosomal marker Tetraspanin8 (Tspan8)20. Tetraspanin-integrin complexes modulate extracellular matrices, adhesion, and exosomal integration into target cells21. Tumour exosome adhesion when antagonising, or knocking down CD44v6, Tspan8, or tetraspanins, prevented carcinogenic spread22.
Other facilitators of organotropism by tumour exosomes include chemokines, CCR7 and CXCR4, and their respective ligands CCL21 and CXCL12. Organ-specific adhesion and metastatic factors have also been identified, for example in bone-metastasising exosomes which include bone-related integrins like periostin and tenascin23. Breast cancer cells were observed homing to natural metastatic sites, lung and bone in murine models when exosomes, with integrins α6β4 and α6β1 from breast cancers, were injected23. This organotropic model is even more convincing when metastases is blocked using integrin-inhibiting mAbs and integrin blocking peptides13,24.
Exosome Influence on Growth and Angiogenesis
During carcinogenesis, the rapid formation and growth of tumour cells often results in hypoxic conditions, forcing tumour cells to modulate their activity from anaerobic respiration using the Warburg effect, as well as secreting exosomes25. Tumour cells often induce a pro-tumour microenvironment through the production and release of angiogenic modulators26. Exosomes released by hypoxic tumour cells secrete angiogenin, vascular endothelial growth factor (VEGF), and colony stimulating factors that induce vascularisation of the tumour milieu and establish a self-sustaining blood supply for continued carcinogenesis. Chan et. al. have also shown that exosomes from nasopharyngeal carcinoma contained pro-angiogenic proteins such as adhesion molecule ICAM-1, as well as CD44, which induced angiogenic responses in recipient endothelial cells27.
Tumour exosomal secretion of Matrix Metallo-Proteinases (MMPs), such as MMP13 and MMP14, and cysteine proteases induce extracellular matrix breakdown and both facilitate tumour angiogenesis and metastasis, and also correlate tumour invasiveness28. Exosome surface expression of Transforming Growth Factor-β (TGF-β), independent of normal TGF-β signalling, triggered fibroblast FGF2 upregulation, and facilitated metastatic spread. Inhibiting Rab27a, a chaperone protein on TGF-β tumour exosomes, resulted in a failure of stroma-assisted tumour growth in vivo29.
Tumour Exosome Immune Evasion and Suppression
Exosomes are important mediators of immune cell communication, and regulators of adaptive immunity28. Dendritic cell exosomes activate B and T cell, and have been shown to have tumour antigen presenting capacity9. However, tumour exosomes also function to suppress and avoid detection by the immune system16. Battke et. al. determined that tumour exosomes secrete immunosuppressive factors, such as IL-10 and PGE-2, downregulating immune response and detection of tumour cells30. Some tumour-derived exosomes express Fas ligand and TNF-α, inducing T cell apoptosis, while other exosomes induce downregulation of NK Group-2 Member-D (NKG2D), inhibiting NK cell proliferation9. Tumour exosomes can also suppress tumour immune surveillance via heat-shock proteins, STAT activation, or production of cytokines through TLR2-MyD88 pathways31, or inducing IL-6 release which impairs dendritic cell maturation9.
Tumour Exosome Therapy Evasion
Tumour exosomes from HER2-overexpressing breast cancer cells, have active anti-chemotherapy effects, by capturing and inactivating the chemotherapy drug trastuzumab in patient serum8. Similarly, exosomes have been shown to express and transfer p-glycoprotein, the multi-drug resistant transporter and can exosomally mediate exportation of cisplatin in drug-resistant ovarian carcinomas32. Exosomal expression of p-glycoprotein correlates with carcinogenesis, and drug failure in ovarian and breast malignancy8,32.
EXOSOMES AND A LIQUID BIOPSY: NOVEL DIAGNOSTIC AND PROGNOSTIC BIOMARKERS
The search for non-invasive diagnostic and prognostic tools has been steadfastly sought to improve early detection and treatment, and recent successes with circulating tumour DNA are promising33. Exosomes, too, are another novel and conceivable non-invasive biomarker in cancer9. Exosomes have been identified in serum and supernatant in vitro and are present in many, possibly all, biological fluids in vivo6. The ability to detect exosomes, by ultracentrifugation, in biological fluid samples makes them an attractive biomarker.
Exosomes from Ewing sarcomas contained 12 overexpressed genes, with intact mRNA, that notably was also resistant to RNase found in human plasma, reaffirming both the sarcoma mRNA stability, but also the potential utility as a diagnostic biomarker34. In high mortality cancers, such as lung cancer, early diagnostic markers are urgently required17. Munagala et. al. found 77 exosomal miRNAs in lung cancer cells that were upregulated or downregulated in primary and secondary lung cancers and further isolated two miRNAs (miR-155 and miR-21) that were specifically upregulated in recurrent lung cancer35. A study assessing a multitude of different adenocarcinomas found significant downregulation of certain exosomal miRNAs, in particular in oesophageal cancers, with diagnostic correlation36. Other studies have shown convincing evidence that exosomes have significant diagnostic potential in prostate, ovarian, colorectal and pancreatic cancers, and in glioblastoma.
Rodríguez et. al. found nucleic acids from breast cancer exosomes in vitro were suitable markers for the prognosis in this patient cohort and the exosomal markers matched a “stemness and metastatic signature” correlating with clinical outcome37. miRNAs from oesophageal carcinomas, glioblastomas and ovarian cancers have shown increased levels of miRNA-21 that correlate to both tumour progression and aggressiveness17. The ovarian cancer study also indicated that of 8 altered miRNAs unique to ovarian cancer exosomes, all correlated with prognostic factors, but 4 were also linked with specific therapeutic resistance38. miR-141 is not only diagnostic in prostate cancers, but the serum levels are a stratification marker of patients with and without metastatic disease9.
Currently, most diagnostic and prognostic exosomal biomarkers have focused on nucleic acids due to their ability to carry cancer specific mutations, increasing diagnostic potential18,39. However, continued apprehensions about how exosomes are classified and identified remain a concern across the literature for diagnostic purposes, especially considering the numerous other similarly sized vesicles, isolation difficulties, specificity of exosomal markers and outstanding unknowns8. Interestingly, recent investigations have begun profiling plasma exosomes in cancer patients and healthy donors using next-generation-sequencing to develop large profile libraries of exosomal tumour biology, with the potential for screening, diagnosis, and potentially sub-typing tumours40.
THERAPEUTIC POTENTIAL OF EXOSOMES
Exosomes have been eagerly investigated for both anti-carcinogenic potential and for the possible use of exosomes themselves as a delivery mechanism for treating neurological pathologies, inflammatory conditions and cancer41. Kosaka et. al. proposed a method, termed ‘exocure’ for the delivery of exogenously developed and intravenously administered tumour-suppressive miRNAs to target sites using exosomes coated with cancer tropisms42.
Wang et. al. have reviewed the drug delivery potential and limitations of exosomes, and have addressed the ongoing research that aims to specifically cargo load exosomes with anti-cancer agents (including miRNAs, doxorubicin, paclitaxel and other tumour-inhibitory factors), and modify exosomes for specifically targeting cancer stem-cells to reduce resistance41. Indeed, in murine models, some multi-drug resistant cancers have responded well to exosome-delivered paclitaxel in lung cancers43, and exosomes encapsulating doxorubicin have shown promise in targeted tumour inhibition in breast cancer cells, in vitro and in vivo44. The targeting by exosomes was achieved by tagging the exosomal surface with lysosome-associated membrane glycoprotein 2b (Lamp2b) with iRGD, a tumour homing peptide, an example of a tumour tropism, that binds αv integrin found on the breast tumour cells44.
CD47 is a “don’t eat me signal”45, that interacts with dendritic cells and macrophages, and can be upregulated and overexpressed in tumours to avoid phagocytosis and identification by the immune system. Single-Regulatory-Protein-α (SIRPα) is a ligand that binds and antagonizes CD47, blocking the evasion from the immune response and phagocytosis. Exosomes, tagged with SIRPα, have been used as a therapy to inhibit cancer immune evasion, and this treatment both in vitro and in vivo lead to an innate and adaptive anti-tumour response, increasing tumour cell phagocytosis and clearance45. Other efforts to inhibit tumour growth include inducing apoptosis selectively in cancer cells, which has been effective in vitro using exosome delivered mutant versions of survivin that induces mitochondrial apoptosis cascades in recipient cells41.
The growing interest in the use of exosomes as a delivery mechanism has some limitations, including concerns about bio-distribution, minimal localised tumour accumulation and rapid clearance of intravenously administered exosomes that was seen in mouse models46. To reduce host immune-clearance of exosomes, Tian et. al. hypothesised that exosomes used for drug administration should be derived from immune cells, such as dendritic cells, to minimise immune-recognition of exogenously administered exosomes44. While exogenous exosomes may be cleared by the immune system, some research hopes to use exosomes for therapeutic intervention in cancer, by way of inoculation, developing exosome-based cancer vaccines10, which relies on the host immune recognition, and clearance and response to the exosomes containing inert tumour nucleic acids47. The most novel of such methods is an exosome-based tumour capturing mechanism called “M-Trap”, using a peritoneally implanted scaffolding device embedded with tumour-attracting exosomes to capture metastatic tumour cells in the peritoneal cavity, which has been efficacious in reducing metastases in murine models of ovarian cancer48. However, until exosomes can be safely constructed, with low immunogenicity and clearance rates, and administered with preferential accumulation in cancer cells in human models, their use in the clinical setting remains foreseeable, but in the future42.
CONCLUSION: A FRESH AND INTRIGUING OUTLOOK FOR EXOSOMES
The remaining unknowns concerning the physiological role of exosomes in health and their dysfunction in disease still requires elucidation. However, the discovery and characterisation of exosomes in relation to tumour progression and metastases has profound scientific, therapeutic and clinical significance. Once the heterogeneous pools of exosomes found in bodily fluids are characterised, identified, and isolated with confidence and replicable rigor49, and clearance mechanisms of exosomes are controlled for, the vast potential for the continued understanding of carcinogenesis and tumour metastases can be tackled with exosomal screening, diagnostic and prognostic tests, and targeted drug delivery47.
References
01. Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: From biogenesis and secretion to biological function. Immunol Lett. 2006;107(2):102–8.
2. Hsu C, Morohashi Y, Yoshimura SI, Manrique-Hoyos N, Jung S, Lauterbach MA, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189(2):223–32.
3. Yang N, Li S, Li G, Zhang S, Tang X, Ni S, et al. The role of extracellular vesicles in mediating progression, metastasis and potential treatment of hepatocellular carcinoma. Oncotarget. 2016;8(2):3683–95.
4. Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–79.
5. Raposo G, Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373– 83.
6. You Y, Shan Y, Chen J, Yue H, You B, Shi S, et al. Matrix metalloproteinase 13-containing exosomes promote nasopharyngeal carcinoma metastasis. Cancer Sci. 2015;106(12):1669–77.
7. Bell E, Taylor MA. Functional Roles for Exosomal MicroRNAs in the Tumour Microenvironment. Comput Struct Biotechnol J. 2017;15:8–13.
8. Pol E Van Der, Bo AN. Classification, Functions , and Clinical Relevance of Extracellular Vesicles. 2012;64(3):676–705.
9. Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126(4):1208–15.
10. Cho J ah, Lee YS, Kim SH, Ko JK, Kim CW. MHC independent anti-tumor immune responses induced by Hsp70-enriched exosomes generate tumor regression in murine models. Cancer Lett. 2009;275(2):256–65.
11. Zakharova L, Svetlova M, Fomina AF. T Cell Exosomes Induce Cholesterol Accumulation in Human Monocytes Via Phosphatidylserine Receptor. 2006;(October):174–81.
12. Christianson HC, Svensson KJ, van Kuppevelt TH, Li J-P, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci U S A. 2013;110(43):17380–5.
13. Arena GO, Arena V, Arena M, Abdouh M. Transfer of malignant traits as opposed to migration of cells: A novel concept to explain metastatic disease. Med Hypotheses. 2017;100:82–6.
14. Skog J, Wurdinger T, Rijn S Van, Meijer D, Gainche L, Sena-esteves M, et al. Glioblastoma microvesicles transport RNA and protein that promote tumor growth and provide diagnostic biomarkers. 2008;10(12):1470–6.
15. Pfeffer S, Grossmann K, Cassidy P, Yang C, Fan M, Kopelovich L, et al. Detection of Exosomal miRNAs in the Plasma of Melanoma Patients. J Clin Med. 2015;4(12):2012–27.
16. Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol. 2015;40:72–81.
17. Reclusa P, Sirera R, Araujo A, Giallombardo M, Valentino A, Sorber L, et al. Exosomes genetic cargo in lung cancer: a truly Pandora’s box. Transl lung cancer Res. 2016;5(5):483–91.
18. Bullock MD, Silva AM, Kanlikilicer-unaldi P, Filant J, Rashed MH, Sood AK, et al. Exosomal Non-Coding RNAs: Diagnostic, Prognostic and Therapeutic Applications in Cancer. 2015;(June):53–68.
19. Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, et al. Systemically Injected Exosomes Targeted to EGFR Deliver Antitumor MicroRNA to Breast Cancer Cells. Mol Ther. 2012;21(1):185–91.
20. Wang Z, Au A von, Schnölzer M, Hackert T, Zöller M, Wang Z, et al. CD44v6- competent tumor exosomes promote motility, invasion and cancer-initiating cell marker expression.... Oncotarget. 2016;5(0).
21. Yue S, Mu W, Erb U, Zöller M. The tetraspanins CD151 and Tspan8 are essential exosome components for the crosstalk between cancer initiating cells and their surrounding. Oncotarget. 2015;6(4):2366–84.
22. Liu D, Li C, Trojanowicz B, Li X, Shi D, Zhan C, et al. CD97 promotion of gastric carcinoma lymphatic metastasis is exosome dependent. Gastric Cancer. 2016;19(3):754–66.
23. Weidle UH, Birzele F, Kollmorgen G, Rüger R. The Multiple Roles of Exosomes in Metastasis. Cancer Genomics Proteomics. 2017;14(1):1–15.
24. Hoshino A et. al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–35.
25. Li L, Li C, Wang S, Wang Z, Jiang J, Wang W, et al. Exosomes derived from hypoxic oral squamous cell carcinoma cells deliver miR-21 to normoxic cells to elicit a prometastatic phenotype. Cancer Res. 2016;76(7):1770–80.
26. Park JE, Tan H Sen, Datta A, Lai RC, Zhang H, Meng W, et al. Hypoxic Tumor Cell Modulates Its Microenvironment to Enhance Angiogenic and Metastatic Potential by Secretion of Proteins and Exosomes. Mol Cell Proteomics. 2010;9(6):1085–99.
27. Chan YK, Zhang H, Liu P, Tsao SW, Lung ML, Mak NK, et al. Proteomic analysis of exosomes from nasopharyngeal carcinoma cell identifies intercellular transfer of angiogenic proteins. Int J Cancer. 2015;137(8):1830–41.
28. Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM. Exosome mediated communication within the tumor microenvironment. J Control Release. 2015;219:278–94.
29. Webber J, Spary L, Sanders A, Chowdhury R, Jiang W, Steadman R, et al. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene. 2015;34(3):319–31.
30. Battke C, Ruiss R, Welsch U, Wimberger P, Lang S, Jochum S, et al. Tumour exosomes inhibit binding of tumour-reactive antibodies to tumour cells and reduce ADCC. Cancer Immunol Immunother. 2011;60(5):639–48.
31. Chow A, Zhou W, Liu L, Fong MY, Champer J, Van Haute D, et al. Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-κB. Sci Rep [Internet]. 2014;4:5750.
32. Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, Naerdemann W, et al. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. 2005;4(October):1595–605.
33. Butler TM, Spellman PT, Gray J. Circulating-tumor DNA as an early detection and diagnostic tool. Curr Opin Genet Dev. 2017;42:14–21.
34. Min L, Shen J, Tu C, Hornicek F, Duan Z. The roles and implications of exosomes in sarcoma. Cancer Metastasis Rev. 2016;35(3):377–90.
35. Munagala R, Aqil F, Gupta RC. Exosomal miRNAs as biomarkers of recurrent lung cancer. Tumor Biol. 2016;37(8):10703– 14.
36. Warnecke-Eberz U, Chon SH, Hölscher AH, Drebber U, Bollschweiler E. Exosomal onco-miRs from serum of patients with adenocarcinoma of the esophagus: comparison of miRNA profiles of exosomes and matching tumor. Tumor Biol. 2015;36(6):4643–53.
37. Rodríguez M, Silva J, Herrera A, Herrera M, Peña C, Martín P, et al. Exosomes enriched in stemness/metastatic-related mRNAS promote oncogenic potential in breast cancer. Oncotarget. 2015;6(38):40575–87.
38. Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110(1):13– 21.
39. Soung Y, Ford S, Zhang V, Chung J. Exosomes in Cancer Diagnostics. Cancers (Basel). 2017;9(1):8.
40. Domenyuk V, Zhong Z, Stark A, Xiao N, O’Neill HA, Wei X, et al. Plasma Exosome Profiling of Cancer Patients by a Next Generation Systems Biology Approach. Sci Rep. 2017;7:42741.
41. Wang J, Zheng Y, Zhao M. Exosome- Based Cancer Therapy: Implication for Targeting Cancer Stem Cells. Front Pharmacol. 2017;7(January):533.
42. Kosaka N, Takeshita F, Yoshioka Y, Hagiwara K, Katsuda T, Ono M, et al. Exosomal tumor-suppressive microRNAs as novel cancer therapy. “Exocure” is another choice for cancer treatment. Adv Drug Deliv Rev. 2013;65(3):376–82.
43. Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine Nanotechnology, Biol Med. 2016;12(3):655–64.
44. Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials [Internet]. 2014;35(7):2383–90.
45. Koh E, Lee EJ, Nam G-H, Hong Y, Cho E, Yang Y, et al. Exosome-SIRPα, a CD47 blockade increases cancer cell phagocytosis. Biomaterials. 2017;121:121–9.
46. Smyth T, Kullberg M, Malik N, Smith- Jones P, Graner MW, Anchordoquy TJ. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release. 2015;199:145–55.
47. Zhang X, Pei Z, Chen J, Ji C, Xu J, Zhang X, et al. Exosomes for immunoregulation and therapeutic intervention in cancer. J Cancer. 2016;7(9):1081–7.
48. De La Fuente A, Alonso-Alconada L, Costa C, Cueva J, Garcia-Caballero T, Lopez-Lopez R, et al. M-Trap: Exosome- Based Capture of Tumor Cells as a New Technology in Peritoneal Metastasis. J Natl Cancer Inst. 2015;107(9):1–10.
49. Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A